Source¶
An evidence item summarizes data from one specific source publication.
Understanding Source¶
Each Evidence Item must be associated with a Source Type and Source ID, which link the Evidence Item to the original publication supporting evidence claims. Currently, CIViC accepts publications indexed in PubMed or abstracts published through the American Society of Clinical Oncology (ASCO) or the American Society of Hematology (ASH). If a PubMed Source Type is selected, the curator can then enter the PubMed ID, which can be verified by comparing the desired source to the abbreviated citation that is automatically generated below the PubMed ID field. Additionally, ClinicalTrials Registry Number(s) are automatically linked via the PubMed database, when available. If an ASCO Source Type is selected, the ASCO Web ID should be entered into the source ID field. If an ASH Source Type is selected, the ASH DOI should be entered into the source ID field.
Curating Source¶
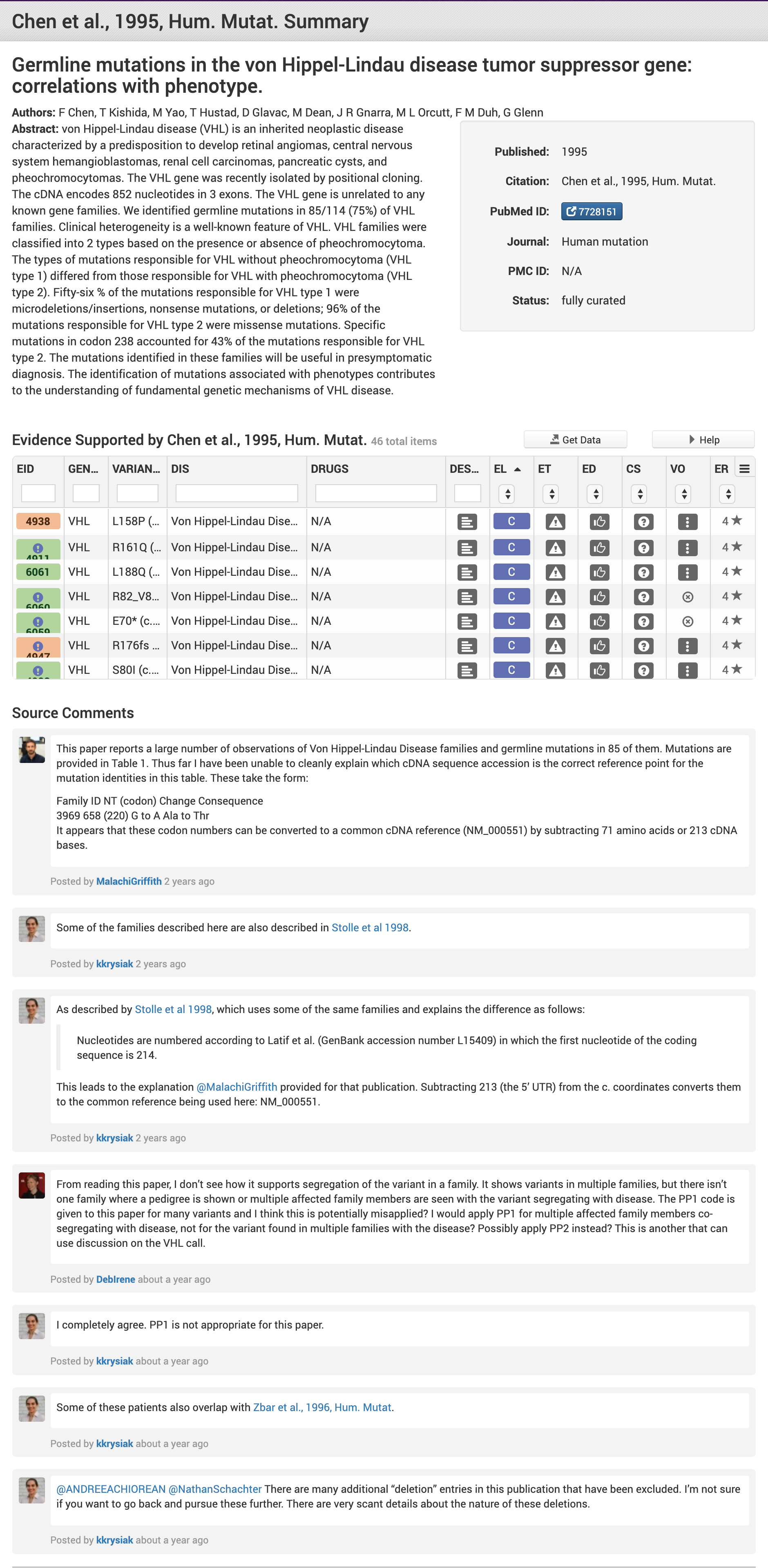
All curated CIViC Evidence, Assertions, Molecular Profile Descriptions, and Gene Descriptions are based on cited sources from the biomedical literature, currently limited to those publications indexed in PubMed, the ASCO Meeting Library, or ASH Meeting Abstracts in the Journal Blood. As soon as any publication is cited in CIViC it appears as a separate Source Record and can be browsed or searched within the CIViC knowledgebase. Each Source Record includes details about the publication as well as a table linking to all CIViC evidence curated for that source.
Comments can be added to any Source Record at any time (see Figure 1 below). Source comments are a useful place to document general or specific notes to aid current and future curation. For example, if a source has an overlapping patient population with another study already curated within CIViC, this can be documented. In other cases, the reference sequence used for determining the CIViC Variant’s coordinates might be recorded.
The current Sources associated with Evidence, Assertions and other information in CIViC can be explored on the CIViC Sources Page.