Evidence Overview¶
The following figure shows the attributes of a CIViC Evidence Item, and the options or values available for each.
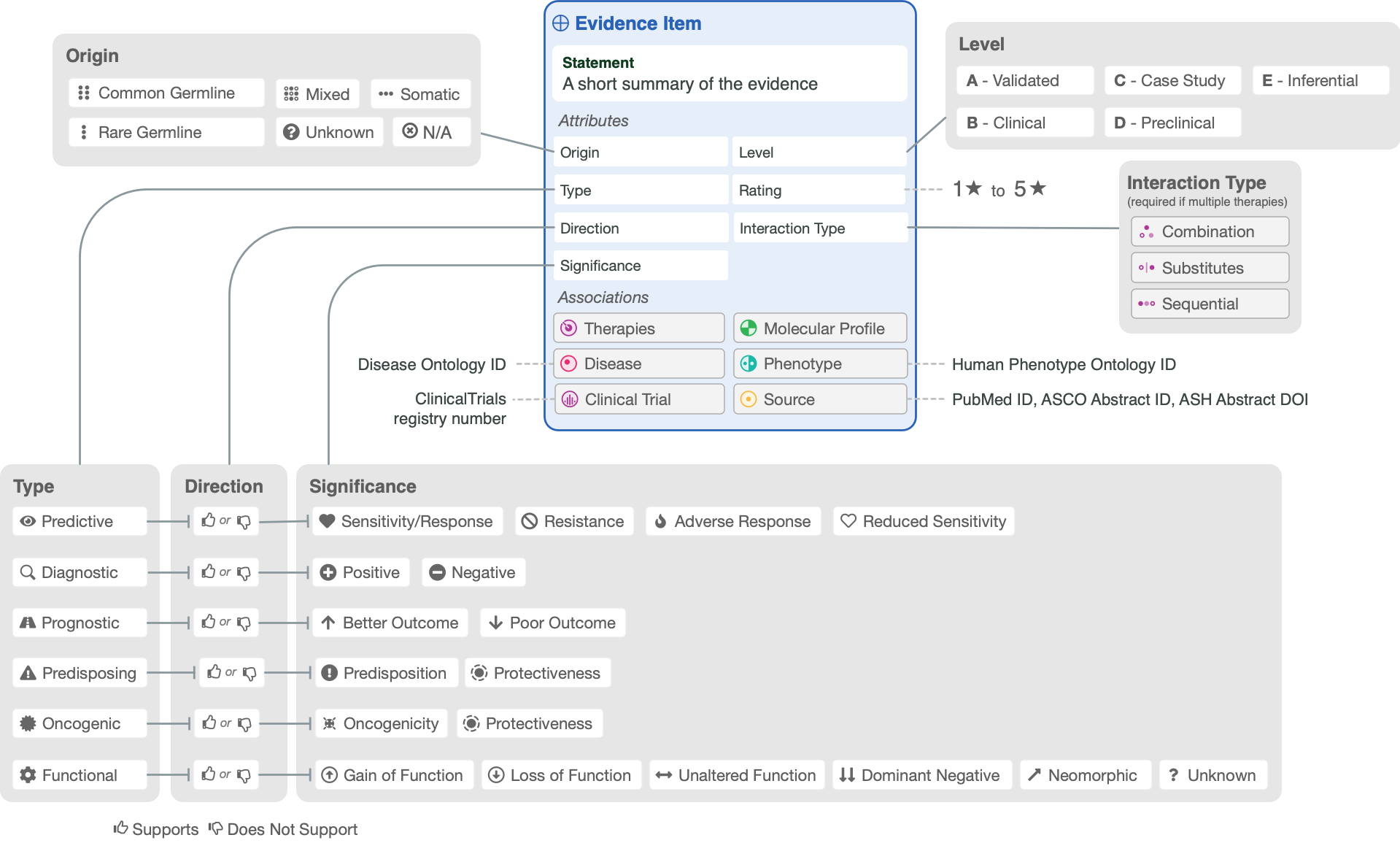
The rows in the following table describe the minimal components of a CIViC Evidence Item. Several Evidence Statements are synthesized at the Molecular Profile (Variant) level into a Molecular Profile Description. However, each Evidence Statement is directly linked to a single article in PubMed or ASCO/ASH abstract. More specific guidelines about Evidence Item components can be found in the additional sections outlined in the table of contents.
Evidence Attributes
Attribute |
Description |
Example |
Source |
|---|---|---|---|
Gene and Genomic event(s)/mutation(s) (e.g., Single nucleotide variant, Insertion/deletion, Gene fusion, Copy number variant, etc.) implicated. May be a simple comprised of a single variant or a more complex combination of variants. |
ESR1 Y537S |
CIViC |
|
Human readable interpretation. Free-form text summary of this molecular profile’s potential clinical interpretations. This interpretation is the synthesis of all other information about the alteration(s) and its clinical relevance and should be the living product of active discussion. |
In this study of 178 non-small cell lung cancer patients, the appearance of EGFR T790M mutation led to resistance to gefitinib. |
CIViC |
|
The type of experiment from which the evidence is curated. From inferential, to proven association in clinical medicine. Refer to the additional documentation on evidence levels for definitions of the five levels allowed in CIViC: validated, clinical, pre-clinical, case study, and inferential. |
Level B - Clinical Evidence. |
CIViC |
|
Category of clinical action/relevance implicated by event. Refer to the additional documentation on evidence types for details on how to enter evidence of each of the six types: Predictive, Prognostic, Diagnostic, Predisposing, Oncogenic and Functional. See ‘Evidence Type’ tab for more information. |
Predictive - The molecular profile (one or more variants) is predictive of sensitivity or resistance to a therapeutic. |
CIViC |
|
An indicator of whether the evidence statement supports or refutes the significance of an event. See ‘Evidence Type’ tab for more information. |
Supports - the evidence supports the significance. |
CIViC |
|
The impact of the molecular profile (one or more variants) for predictive, prognostic, diagnostic, oncogenic or functional evidence types. See ‘Evidence Type’ tab for more information. |
Resistant or Non-response - mutation is associated with resistance to therapy. |
CIViC |
|
Presumed cellular origin of the Variant in samples from the literature citation where the effect of this Variant is being evaluated. |
Somatic |
CIViC |
|
Specific disease or disease subtype that is associated with this event and its clinical implication. Links directly to Disease Ontology. |
Estrogen-receptor positive breast cancer (DOID: 0060075). |
CIViC (Disease Ontology) |
|
Specific phenotypes associated with the evidence statement. |
Pancreatic cysts (HP:0001737). |
The Human Phenotype Ontology (HPO) |
|
For predictive evidence, indicates the therapy for which sensitivity or resistance is indicated (With NCIt ID if available). |
Tamoxifen, Raloxifene (NCIt IDs: C62078, C62078). |
CIViC (NCIt) |
|
Therapy Interaction Type |
For predictive evidence involving more than one Therapy, specifies the relationship between the therapies (usually drugs) by indicating whether they are Subtitutes for each other or are used in Sequential or Combination treatments. |
Substitutes - The therapies listed are often considered to be of the same family, or behave similarly in a treatment setting. |
CIViC |
Citation |
Publication where the event was described/explored automatically generated from curator-provided PubMed ID and links to internal CIViC publication page showing all Evidence Items from the publication. |
Toy et al., 2013, Nat. Genet. (PMID: 24185512) |
CIViC (PubMed) |
PubMed ID |
PubMed ID for publication where the event was described/explored with direct link to PubMed. |
24185512 |
CIViC (PubMed) |
Clinical trial associated with the evidence item. |
NCT01154140 |
ClinicalTrials.gov |
|
A rating on a 5-star scale, portraying the curators trust in the experiments from which the evidence is curated. Refer to the additional documentation on trust ratings for guidance on how to score an evidence item. |
5-stars - Strong, well supported evidence from a lab or journal with respected academic standing. Experiments are well controlled, and results are clean and reproducible across multiple replicates. |
CIViC |